Gamma Digital’s Production Support System manages logistic processes from production planning through production scheduling to the loading of finished products, tracking and recording material movement, and managing remnants.
It continuously monitors the status of the production line, records machine positions and runtime, and alerts users when maintenance is due.
Produce production documentation in electronic and printable format to comply with FDA regulations. The system’s flexible and easy-to-use graphical interfaces visualize every major step in the manufacturing process (production schedule, shift scheduling, machine condition), making production easier daily work of all employees.
The system cooperates with enterprise management software, and the statistics it produces help guide the management decisions.
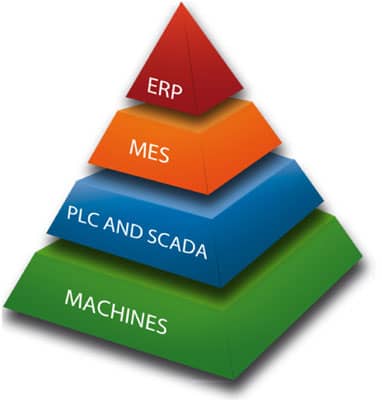
MAIN FEATURES OF THE SYSTEM
- Production planning and scheduling support
- ERP system integration
- Lottery support
- Automatic buffer management
- Registration of material consumption
- Finished goods registration
- Maradékkezelés
- Managing common and roll-over materials
- Production of production documentation in electronic and printable form
- In Process Control (IPC) support
- Machine and working hours records and accounting support
- Support for cleanroom production control and tracking with panel PC client
- GAMP and FDA compliance
- Working time records
- Visual production scheduling interface
- Dokumentumkezelés
- Management of multiple production lines simultaneously
- In-production control, integrated IPC
- Process accounting, residue management
- Electronic manufacturing documentation
System Benefits
Flexibility: Gamma Digital’s Production Support System is fully configurable to the production environment, and our development engineers build the system according to the production process. MES supports production-related devices, barcode scanners and printers, PDAs and PocketPCs, AGVs and PLC-controlled devices.
Integration: Our production support system can be securely integrated into the ERP system and the enterprise management system works better if it is built with manufacturing software. Standard drivers for our system are also accepted by SAP.
Compliance: Complies with U.S. Food and Drug Administration: CFR Title 21 Part 11 Electronic records; Electronic Signatures. Conforms to EudraLex – Volume 4 Good Manufacturing Practice (GMP) Guidelines.
Direct Support: Gamma Digital also provides development and technical support for the high level integration and excellent operation of the MES system. Thanks to our direct support system and intensive change cycle management, we respond quickly to bug reports and user needs. We are available 24 hours a day, seven days a week. We provide support for our software throughout the product life cycle.

